
NFPA and other certifying agencies have shifted their approach from the inspection and certification of hospitals from an occupancy-based code to a risk-based philosophy.
This requires the hospital to identify potential dangers and faults in all their systems and have on hand a written policy to handle equipment maintenance and failures.
This plan is Risk Assessment.
Are You Struggling With Medical Gas Compliance?
CHT offers medical gas services to help you reach your compliance goals. To help you navigate through these challenges, start with a free 30-minute discovery call.

Risk Management: Part of the Higher Plan
“Risk management is a structured approach to managing uncertainty related to a threat through a sequence of human activities, including risk assessment, strategy development to manage risk, and mitigation of risk using managerial resources.
The strategies could include transferring the risk to another party, avoiding the risk, reducing the negative effect, and accepting some or all of the consequences of a particular risk.”
Several surveyors are asking for or requiring Risk Assessment plans as part of their inspection routine. Other than the general principles outlined here, specific detailed guidelines for equipment, processes, or buildings are still being formulated.
Risk assessments generally ask:
-
What can go wrong?
-
How bad could it be?
-
What action should be taken?
-
How often might it happen?
-
How do we avoid this problem in the future?
6 Steps to Provide a Remedy for an Identified Risk
There are six specific steps to carrying out a risk assessment to achieve the following:
- Identify the hazards
- Identify who or what could be injured
- Evaluate the risk
- Evaluate probability
- Record and identify parties included
- Review and inform
Step 1: Research. Identify what failures or incidents can cause damage.
Step 2: Identify who or what could be injured or displaced by such a failure or event: patients, staff, or the public.
Step 3: How critical would the damage be if the defect took place?
Step 4: How likely is this problem to happen?
Step 5: Create a remedy or procedure to work around the adverse event and identify the responsible parties.
Step 6: Review the corrective action plan with staff to make sure they understand and can fulfill their responsibilities in the event of a defect occurring.
The events you identify should be filtered through a grid like the one below that clarifies the importance and urgency of action to remedy the problem and value the risk of a bad outcome.
Immediate Threat to Life
Source: The Joint Commission
List Equipment to Prepare for Remedies
ASHE provides a spreadsheet to list equipment and areas of the hospital that are important to patient care. From there, you can individually plan the remedies for failures that may occur at these sites.
NFPA, CMS, and TJC require an inventory of equipment to be available during the inspection, and for medical gas purposes, that would be the starting point of filling out the equipment grid from ASHE.
Risk Assessment Form Example
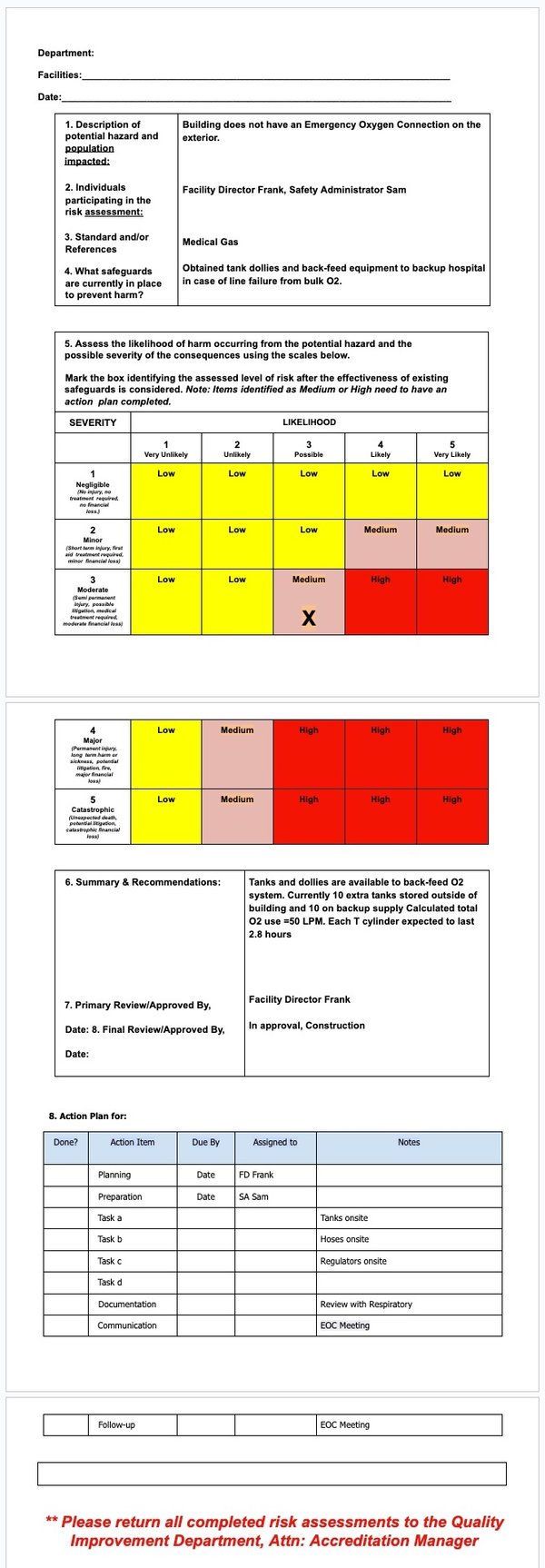
Those hospitals using the Vitaleyez software should be able to get the inventory identification information directly from the program. (i.e., asset description, manufacturer, model #, and all historical inspection data.)
Hospital Risk Assessment Defined by Risk to Patient
Risk-based assessment assigns a value to each asset by their use and their potential to be harmful to the patients or staff. For example, an oxygen outlet in an emergency room would have the highest urgency, while the outlet in the storeroom would have the lowest priority.
The hospital, in conjunction with the inspecting company following NFPA guidelines, would establish an urgency level for each device and then set an inspection and repair schedule for the assets based on the risk rating to patients rather than the date on the calendar.
Building System Categories Defined by Threat
The categories are broken down into harm to patients, staff, and visitors.
- Failure may cause death or serious injury
- Failure limited to minor injuries
- Failure may cause discomfort
- No impact on patients or caregivers
source: whea.com
Category 1 - The system must always be highly functional. An example in this category would be a medical gas system in an ICU.
Category 2 - A system failure would not involve risk to life. High dependability is expected in this category.
Category 3 - Failure would cause discomfort but is not likely to cause injury.
Category 4 - Patients are not adversely affected.
All building system categories will be driven by the ensuing risk assessment procedures.
Maintenance Programs and Risk Assessment
The Joint Commission identifies the activities and frequencies for maintaining, inspecting, and testing all operating components of utility systems.
Various maintenance strategies may be used to ensure reliable performance (i.e., predictive maintenance, reliability-centered maintenance, interval-based inspections, corrective maintenance, or metered maintenance).
Defined intervals may be based on criteria such as manufacturers’ recommendations, risk levels, and current organization experience.
According to Standard EC.02.04.01, the organization manages medical equipment risks. Also, in agreement with the Elements of Performance for EC.02.04.01 A 2, the organization maintains either a written inventory of all medical equipment or a written inventory of selected equipment categorized by the physical risk associated with use (including all life-support equipment) and equipment incident history.
The organization evaluates new types of equipment before initial use to determine whether they should be included in the inventory.
Alternative Equipment Maintenance Programs
TJC and CMS allow Alternative Equipment Maintenance programs (which deviate from the manufacturer’s suggested intervals) if the hospital can prove with historical and industry data that the alternative program will keep up equipment performance. The Risk Assessment, which has become the standard philosophy for assessing hospitals, must support these alternative programs.
“The Joint Commission standards are not prescriptive regarding testing frequencies for piped medical gas and vacuum systems. The facility may determine its testing frequency by policy and the surveyor will evaluate testing based on that policy.
In accordance with EC.02.05.09, for each piped medical gas and vacuum system, the source, distribution, inlets/outlets, and the alarms that protect the systems are to be maintained in a safe and reliable condition.
The organization is required by EC.02.05.01 to develop a maintenance strategy based upon either manufacturer's recommendations or an alternative equipment maintenance (AEM) strategy. Piped medical gas and vacuum systems are considered high-risk utility systems.
Issues such as system complexity, system age/condition, patient acuity, etc. are to be used to assess maintenance strategies. NFPA 99-2012 Appendix B can be used as a guide for establishing a maintenance strategy, but appendix material is not required unless adopted by a controlling authority. The survey process will evaluate the maintenance strategy assessment process for effectiveness and validate that it has been properly implemented.”
Release of TJC Elements of Performance
TJC-accredited hospitals, for example, now face an even more important reason for having an effective AEM-inclusion risk assessment process since the official 1-9-2017 release of TJC’s updated Elements of Performance. The broad utility system inspection, testing & maintenance (ITM) Standard EC.02.05.05 contains newly changed language concerning required utility ITM completion rates.
The impacts of the updated EP language issued on 1-9-2017 basically require:
In EC.02.05.05 EP-4: 100% on-time compliance for all High-Risk utility system components, whether done through the Alternative Equipment Maintenance (AEM) program or done in accordance with manufacturer’s recommendations;
In EC.02.05.05 EP-5: 100% on-time compliance for all Infection Control utility system components, whether done through the AEM program or done in accordance with manufacturer’s recommendations;
In EC.02.05.05 EP-6: down to not less than 90% on-time compliance for other Non-High Risk components done through the AEM program only if the organization’s policy (presumed to be the utility systems equipment AEM Program policy) permits such deferral.
In other words, TJC-accredited hospitals have some flexibility to mandate 100% on-time inspection, testing, and maintenance compliance only for their non-high-risk and non-infection control utility system components that are managed through an acceptable Alternative Equipment Maintenance Program.
Critical Factors to Consider When Evaluating Risks Associated With Equipment
Whether equipment is critical (TJC high-risk) equipment is one of the tests. It may even be the most important factor to consider, but it is not the only factor.
Within the State Operations Manual, Appendix A – Survey Protocol, Regulations and Interpretive Guidelines for Hospitals, A-0724 (Rev.); Interpretive Guidelines §482.41(c)(2), CMS states:
Factors for a hospital to consider when evaluating the risks associated with a particular type of equipment include, but are not limited to:
- How the equipment is used and the likely consequences of equipment failure or malfunction – would failure or malfunction of the equipment hospital-wide or in a particular setting be likely to cause harm to a patient or a staff person?
- How serious is the harm likely to be?
- How widespread is the harm likely to be?
- Information, if available, on the manufacturer’s equipment maintenance recommendations, including the rationale for the manufacturer’s recommendations.
Maintenance Requirements of the Equipment:
- Are they simple or complex?
- Are the manufacturer’s instructions and procedures available in the hospital, and if so, can the hospital explain how and why it is modifying the manufacturer’s instructions?
- If the manufacturer’s instructions are not available in the hospital, how does the hospital assess whether the AEM uses appropriate maintenance strategies?
- How readily can the hospital validate the effectiveness of AEM methods for particular equipment? For example, can the hospital explain how it ensures there is no reduction in the quality of the performance of biomedical equipment subjected to alternate maintenance methods?
- What is the timely availability of alternate devices or backup systems in the event of equipment failure or malfunction?
- Have you considered the incident history of identical or very similar equipment?
CMS also states,
Generally multiple factors must be considered, since different types of equipment present different combinations of severity of potential harm and likelihood of failure. The hospital is expected to be able to demonstrate to a surveyor the factors it considered in its risk assessment for equipment placed in its AEM program.
Legacy computerized maintenance management systems (CMMS) generally do not adequately address these AEM inclusion risk assessment requirements with their original risk assessment programming. This situation appears to be because the newly required risk assessment factors are generally not the same as the more commonly-used factors that hospitals have used in past utility system risk assessments. A new risk assessment framework appears to be required to support AEM-inclusion decisions.
Suggested CMS Checklist to Identify Risks and Their Protections and Remedies
source: CMS.gov
For general hospitals performing anesthesia, the risk level for medical gas equipment is always high as they sustain life, and their interruption would likely cause death or serious harm to patients and perhaps other equipment, except in Category 3 or 4 facilities.
The default inspection and testing regime for medical gas equipment has been the Manufacturer’s suggested frequency. This usually means annually for equipment like outlets, inlets, valves, and alarms, quarterly for active source equipment like pumps and compressors, and monthly for power changeover test systems.
Emergency Operations Planning
Another area that has gained current attention is emergency planning for larger-scale shutdowns that will mean unusually large numbers of patients and damaged utility systems that will strain normal hospital operations.
Here are the suggested questions from one agency:
Emergency Operations Planning
- Life Supporting Equipment
- Oxygen, Medical Air, Vacuum (Potential Systems)
- How will you ensure systems are available during an emergency?
- What is the protocol for monitoring systems during an emergency?
- How will the Emergency Operations Plan be activated/deactivated?
- Will the plan meet the 96-Hour Sustainability Requirement?
- Staff must be educated on their specific duties and responsibilities.
- Some options for contingencies:
- Cylinders available for critical care patients
- Temporary backfeed of all critical care portions of the system
- Portable vacuum systems available for critical care areas
- Suppliers temporary bulk oxygen trailer (Might not be practical)
Conclusion
As the various agencies collaborate on their reporting standards and methods, the responsibility of the hospital to identify and maintain equipment is paramount.
The surveyors may well ask for an inventory of any or all equipment in the hospital, including its maintenance history for at least the past two years. Any equipment failures that lead to serious harm to patients or staff are likely to be scrutinized as the process evolves. Detailed records of defective parts and adjustments need to be recorded as well.
An exhaustive study by John Collins, FASHE, HFDP, done for ASHE Monograph determined the most frequently failing devices leading to patient injury were circuit boards. Assuming this holds true for your hospital, the piped medical gas system should be very reliable as the only circuit boards are in the source equipment control panels and alarm panels.
This could mean more scrutiny of the activation and calibration of alarm systems for medical gas. Motors and wear parts were far behind in terms of creating failures, but many of these potential failures can be anticipated with a regular preventive maintenance program.
The Vitaleyez program for medical gas systems provides the statistical history base for your risk assessment program. With the asset identity details and historical inspection data in hand, it is easier to fill in the grid above to satisfy the inspecting agencies.
CHT offers medical gas services to help you reach your compliance goals. To help you navigate through these challenges, we offer a free 30-minute discovery call.
Note: The ideas, words, and illustrations in this article are a compilation of the wisdom drawn from CMS, TJC, ASHE, NFPA, and the NHS.




