In the United States, medical devices are regulated by the Food & Drug Administration (FDA). Most medical devices are required to follow specific design control processes and comply with FDA 21 CFR part 820 Quality System Regulations.
As a leader in the control, management, and monitoring of medical gas systems, our commitment to the medical field encourages us to stay on top of design controls in medical devices, product development, and risk management.

Let's get started. Below, we will discuss:
- How Medical Devices are Classified
- Medical Device Product Development
- Design Controls as Part of the Product Development Process
- Risk Management
- Document Control and Records Management
- Supplier Management Issues and How to Avoid Them
How Medical Devices are Classified
According to the FDA, "[Medical devices are] assigned to one of the three regulatory classes based on the level of control necessary to assure safety and effectiveness of the device."
The FDA classifies the devices into one of three regulatory classes:
Class I - The lowest level of risk, the lowest level of regulatory control.
Class II - Slightly higher risk than Class I.
Class III - The highest level of risk and the most complex. (These devices usually sustain or support life.)
The classification is based on the risk associated with the device.
Innovatum, the label management system for FDA-regulated Pharmaceutical, Medical Device, and Biotechnology manufacturers, suggests these top questions to determine how a medical device is classified:
- How long will the device be used?
- Is the device surgically invasive?
- Will the device be surgically implanted?
- Does the device contain medicinal substances?
For more on finding the classification of a device:
Understanding the Difference between Class I, Class II and Class III Medical Devices
Medical Device Product Development
Design controls are set to provide the designers and engineers with visibility of the design process. Adhering to this process increases the opportunity to recognize problems at an earlier stage and make the necessary corrections.
There are at least four critical areas to consider in medical device product development:
- Design Controls
- Risk Management
- Document Control (Design History File) & Records Management
- Supplier Management
Design Controls as Part of the Product Development Process
Design controls can be applied at any stage of the product development process. Its goal is to make certain the device meets user needs and its intended purpose. Design controls are part of the larger product development process.
Product development converts an idea into a physical form. Part of this process includes the concept and testing through prototyping. The prototyping methodology allows you to design a working prototype or early sample of your device.
One of these models, the Waterfall Model, is a useful tool when introducing design controls. The Lean and Agile approaches can also be utilized in medical device product development.
Greenlight Guru, a quality management software designed exclusively for the medical device industry, says the real essence of design controls are...
"Proof that you have designed a safe product that meets user needs and requirements."
Jon Speer, founder & vice president of QA/RA and Greenlight Guru, also adds that there are a few design control absolutes that must take place and be documented.
- Design output must include acceptance criteria
- All the parts and pieces that make up your design
- Design output and design inputs have a relationship
- Including design, development and/or requirements
- The design must be validated
- The right product meets the needs of the user
- Design controls must be part of design reviews
- The design is reviewed against its requirements
Design control and risk management work hand in hand.
Risk Management Improves Quality and Safety
Risk management is a necessary part of any product development lifecycle. It identifies, analyzes, and reduces potential product issues.
There are distinct policies, procedures, and practices used to analyze, evaluate, and monitor risks in medical devices.
eInfochips, a leading global provider of product engineering design services, understands the standard steps to implement a thorough risk management lifecycle for medical devices. Their 5-step guide to risk management includes:
- Framework & Planning for Risk Management Medical Devices
- Risk Analysis
- Risk Evaluation
- Risk Control
- Reports and Documents
Risk management improves the overall quality and safety of medical devices.
At CHT, we think of risk management as an approach to managing uncertainties related to a threat through risk assessment. We evaluate the immediate threat to life: the likelihood of harm to a patient, staff, or visitor. The likelihood to harm falls into categories of high (likely), moderate (possible), or low (rare).
The Vitaleyez™ program for medical gas systems provides a statistical history base for your risk assessment program. The dashboard is designed to help healthcare systems manage their medical gas programs more efficiently.
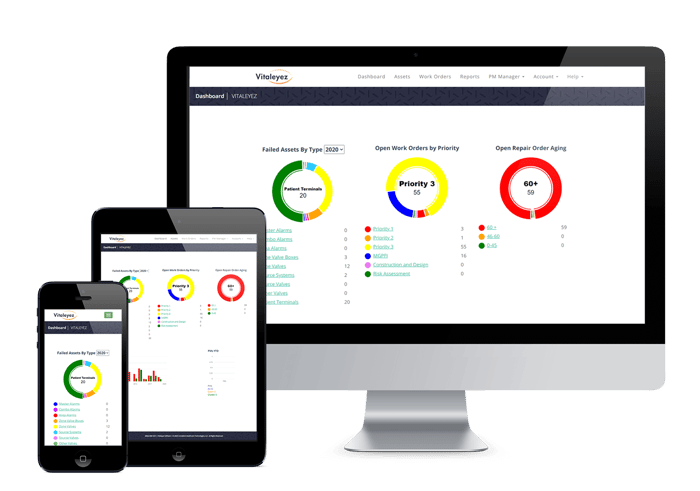
The pie charts on the dashboard of Vitaleyez™, shown above, paint a picture of your efficiency by demonstrating how much work was done and the declining deficiencies year-to-year.
The quality risk with medical devices involves risks to the longevity of the device during the product lifecycle, which includes risk to the patient.
The risk level is broken down into four types; critical, major, minor, and negligible. Critical has the highest potential for causing unsafe conditions, while negligible has no potential for patient injury.
Use risk control to reduce risks, keep patients safe and become more efficient.
Document Control & Records Management
With medical device development, document control, and records management need to be maintained. An effective way to do this is through an overview of the process and changes. It's important to show the history of revisions with its approvals.
A design history file (DHF) is an accumulation of records with the design history from start to finish. It should include identification of the design, date, and individuals performing the review.
"After you launch your medical device, the DHF is important in the event the project is ever audited by the FDA or others." [source]
Having a system in place will eliminate missing documents and records.
Supplier Management Issues and How to Avoid Them
Medical technology manufacturing is a complex process. Medical device manufacturers rely on suppliers that may not fully understand the intricacies involved in the process, design, and manufacturing.
The supplier’s partial understanding could potentially create a problem for the manufacturer because, in the end, the manufacturer is fully responsible for compliance. It is imperative to work with suppliers that are compliant and have a proven track record.
There must be clearly established quality requirements, updated specifications, and suppliers that will participate in the validation process.
Matthew Lowe, executive vice president at MasterControl Inc., offers excellent advice on how to manage this delicate process:
1. Ask for references
2. Establish an audit program
3. Incorporate purchasing controls in your quality management system
4. Be vigilant about outsourcing
MasterControl provides a "full line of industry-specific quality and compliance software solutions and services." This, in turn, enables regulated companies to accelerate its compliance and get to market faster.
One solution is MasterControl's Document Control Software. It organizes and tracks documents, which allows companies to reduce costs and help eliminate audit and compliance difficulties.
MasterControl YouTube. (2015, November 2015) Demo Master Control Document Control Software
Documentation is reviewed at each phase of production. As discussed earlier, the Design History File is an accumulation of records. If the DHF is incomplete, additional time and effort are needed to supply the required information for a review or FDA inspection.
Conclusion
The quality standards of medical devices consist of design control, risk management, document control, and supplier management (at the bare minimum).
At CHT, we continue to provide innovative technology solutions that help facilities strive for a simple, safe, and secure way to manage healthcare compliance and patient safety.




